What is the difference between electronegativity and electron affinity?
3 Answers
Well, they are talking about the same thing but here are the definitions.
Electronegativity is a chemical property that says how well an atom can attract electrons towards itself. The electronegativity of an atom is influenced by the atom's atomic number and the distance between the atom's valence electrons It was first theorised by Linus Pauling in 1932.
The electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule in the gaseous state to form a negative ion.
X + e− →
Electron affinity is a property of an isolated atom in the gaseous state. It is an energy term and is measured in units of joules per mole.
Electronegativity is a property of an atom in a molecule. It can be expressed only in arbitrary dimensionless units with respect to a reference atom.
Electron Affinity
Electron affinity, EA, measures the energy released when an electron adds to a gaseous atom.
It is usually reported as the energy per mole of atoms. For example,
Cl(g) + e⁻ → Cl⁻(g); EA = -349 kJ/mol
Electronegativity
Electronegativity,
Electronegativity cannot be directly measured. It must be calculated from other properties such as bond energies, ionization energies, and electron affinities of the bonded atoms.
This gives a dimensionless quantity on a relative "Pauling scale" that runs from around 0.7 to 3.98. Hydrogen is assigned an arbitrary value of 2.20 "Pauling units".
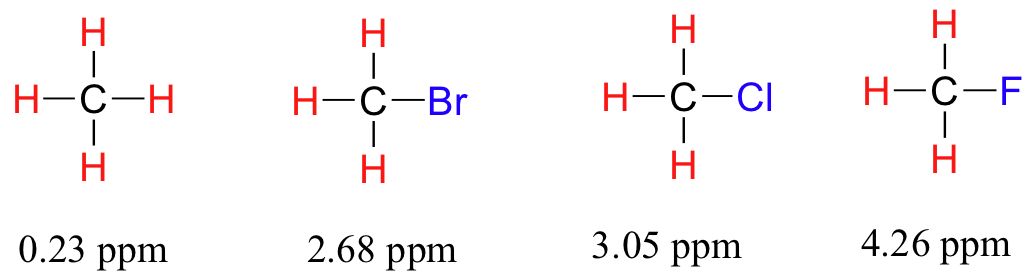
The greater the electronegativity difference
The less electronegative atom will have a decreased electron density.
The protons attached to that atom will be deshielded.
As the electronegativity of the substituent increases, so does the extent of deshielding, and so does the chemical shift.
 chemwiki.ucdavis.edu
chemwiki.ucdavis.edu
The difference in energy of a neutral atom and its anion( Negative ion) in gas phase is the electron affinity (A) . When an electron is added to an atom or molecule, the more energy it releases the more readily an atom becomes into an ion.
A = E(N) - E(N+1) , where N is the no. of electrons in the neutral atom.
Hence , it is a measurable quantity.
On the other hand, Electronegativity is defined as the power(tendency) with which an atom attracts electrons to itself in a molecule or in a covalent bond. Electronegativity is affected by the atom’s atomic number and the distance that its valence electrons reside from the charged nucleus. Hence, higher the electronegativity of a compound or an element, the more it attracts electrons towards it.
Electronegativity deals with individual atoms, while electron affinity deals with atoms in a molecule. Electronegativity values can also change depending on the molecule that it is bonding to, while electron affinity does not change.


