How does electron capture cause transmutations?
1 Answer
May 20, 2014
In electron capture, the electron combines with a proton to make a neutron. This changes the atomic number of the atom, so the process is a transmutation.
Electron capture is a major decay mode for isotopes with too many protons in the nucleus. Since a proton becomes a neutron, the number of protons decreases by 1, but the atomic mass stays the same.
An example of electron capture is the decay of beryllium-7.
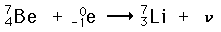
The process also emits a neutrino. The neutrino is often omitted from the equation, because it has no mass or charge. Its only function is to carry away some of the excess energy.
This is a transmutation, because it converts one element to another.

