What is the angular quantum number?
1 Answer
Jun 23, 2014
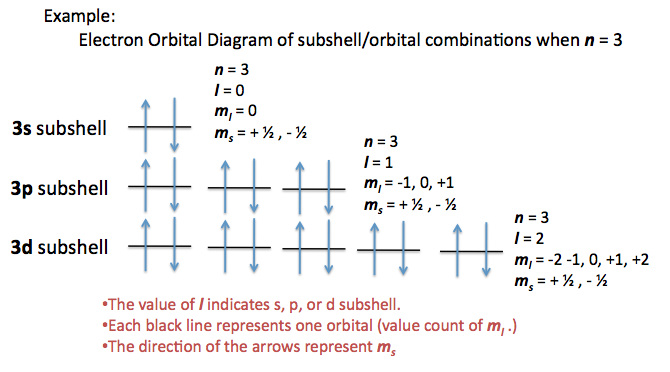
Angular Quantum number describes the subshell/sub energy level....
Quantum numbers can be used to describe the exact location of an electron.
There are four quantum numbers:
-
n - describes the energy level
-
ℓ - describes the subenergy level (0 to n-1 )
- mℓ - describes the orbital ( -ℓ to +ℓ )
- ms - describes the spin (clockwise or anti clockwise : -½ or +½)