Why is the atomic number a whole number?
1 Answer
Jun 18, 2014
The Atomic number is equal to the number of protons present in the atom. Thereby, the atomic number (number of protons ) is a whole number
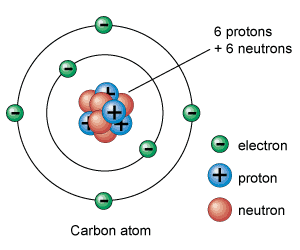
For example, the atomic number of carbon is 6 - this means that all atoms of carbon, no matter what, have six protons. On the other hand, that weirdo oxygen has an atomic number of 8, indicating that oxygen atoms always have 8 protons.
if you want info about how it was discovered visit this page...
https://socratic.org/questions/who-discovered-the-atomic-number