How many valence electrons does hydrogen have?
1 Answer
Hydrogen has 1 valence electron.
Explanation:
Hydrogen is in the first row of the Periodic Table. Elements in the first row are filling their
Since hydrogen is the first element, its electron configuration is
Valence electrons are the outermost electrons or electrons in the largest energy level.
Hydrogen has only one electron, so that electron is a valence electron.
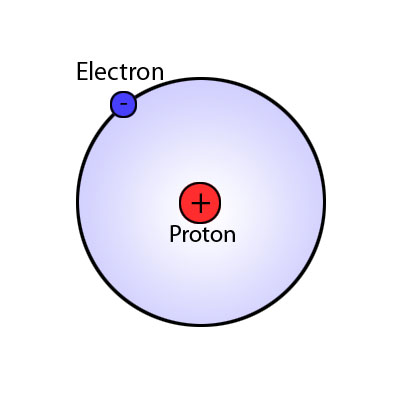
Example
How many electrons does helium have?
Solution
Helium is also in the first row of the Periodic Table. It is filling its 1s orbital.
Since helium is the second element, it has two electrons. Its electron configuration is

Helium has two electrons in its valence shell.
Here is a video that gives further explanation of this topic.
Video from: Noel Pauller


