Does a chlorate ion have resonance structures?
1 Answer
Jul 13, 2014
Yes, the chlorate ion has three major contributors to the resonance hybrid.
We can write three important equivalent Lewis structures for the chlorate ion. See this diagram from http://www.austincc.edu/lgregory/Lewis%20Diagram%20polyatomic%20answers.htm
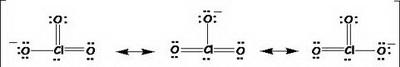
There are other Lewis structures, but they are less important.
Each of the three structures is a resonance contributor.
The structure of the resonance hybrid is
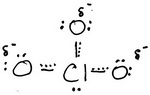
The negative charge is distributed equally among the three O atoms.

