Question #6f12a
1 Answer
Aug 7, 2014
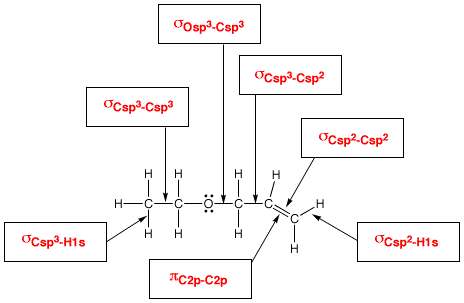
Valence bond (VB) theory assumes that all bonds are localized bonds formed by sharing electrons in overlapping atomic orbitals.
VB theory explains sigma and pi bonds in terms of end-on and side-on overlap of atomic orbitals.
It explains hybridization in terms of mixing of atomic s, p, and d orbitals.
VB theory explains the shape of molecules in terms of VSEPR theory.
In short, VB theory is the theory that you have been learning all along.