What are dehydration synthesis reactions?
1 Answer
Sep 9, 2014
Dehydration synthesis reactions are reactions in which molecules combine by the removal of a H atom and an OH group between them, which together form a molecule of water,
For example, the formation of maltose (malt sugar) from two molecules of glucose is a dehydration synthesis.
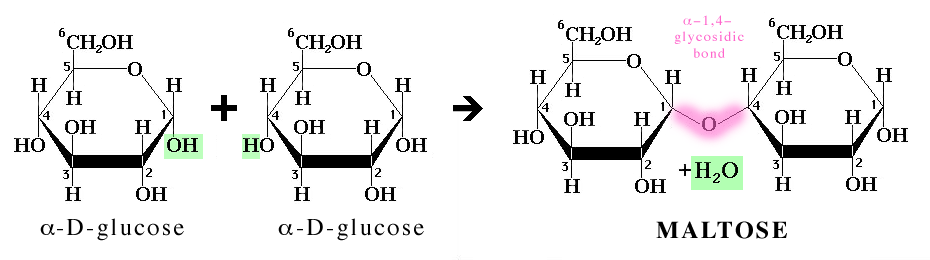
Most biomolecules, such as carbohydrates, triglycerides, proteins, and nucleotides are formed in this way.

