How many NMR signals does CH_3CH_2CH_2OH show?
1 Answer
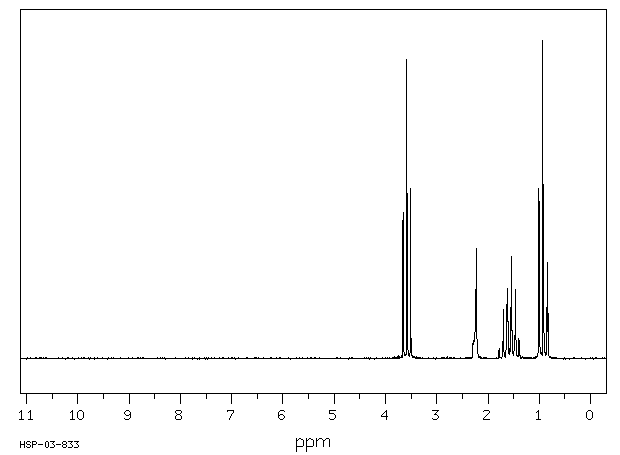
The H NMR spectrum of propan-1-ol shows four signals.
The molecule has no symmetry elements. So the three carbon atoms and the oxygen atom represent four different hydrogen environments.
We should see four signals with area ratios 3:2:2:1.
The OH signal varies from δ 1 to δ 6, depending on the solvent and dilution, so we should expect to see a 1H signal somewhere in this region.
The electronegative O atom pulls all signals downfield. The effect decreases rapidly with distance.
The CH₂ group at C-1 is pulled about 2 ppm downfield from its normal value of
δ 1.4. We should see a 2H signal at about δ 3.4.
The CH₂ group at C-2 is pulled about 0.1 ppm downfield. We should see a 2H signal at about δ 2.5.
The CH₃ group should be at its normal location. We should see a 3H signal at δ 0.9.
All signals except the O-H signal should show spin-spin splitting.
Here is an actual spectrum of propan-1-ol.