How many NMR signals does CH_3CH_2OCH_2CH_3 show?
1 Answer
Oct 28, 2014
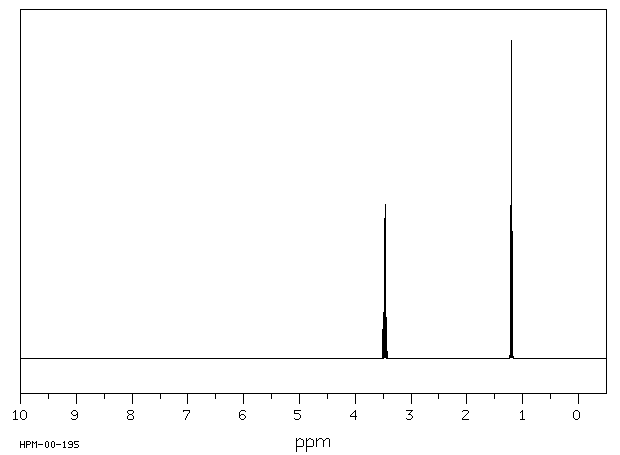
The H NMR spectrum of diethyl ether shows two signals.

The molecule has a plane of symmetry, so the two CH₃ groups are equivalent, and the two CH₂ groups are equivalent.
We should see two signals with area ratios 6:4 or 3:2.
The electronegative O atom pulls all signals downfield. The effect decreases rapidly with distance.
We should expect the CH₂ groups to be pulled about 2 ppm downfield from their normal value of δ 1.4. They should give a 4H signal at about δ 3.4.
We should expect the CH₃ groups to be pulled about 0.3 ppm downfield from their normal value of δ 0.9. They should give a 6H signal at about δ 1.2.
The signals will be split by spin-spin coupling.
Here is a spectrum of diethyl ether.