Why does an sp3-hybridized electrophile must have a leaving group (X) in order for the reaction to take place?
1 Answer
A general theme in organic chemistry is that carbon atoms usually have four bonds. These four bonds can consist of single, double or triple bonds, so long as the sum adds up to four. For
Suppose we consider the following
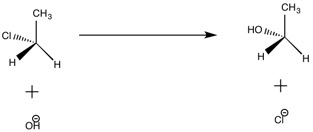
In this reaction, hydroxide is acting as a nucleophile and attacks chloroethane, which acts as the electrophile. Notice that the
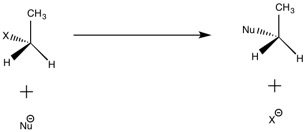
It is important to note that reactions will only proceed when the incoming nucleophile is a worse leaving group (and better leaving group) than the atom already present. Otherwise, there is no driving force to cause the reaction to go.

