How can I draw the skeletal-line structure for 3-octyne?
1 Answer
Let's go through this step by step:
First off, when naming compounds, recognize the longest chain of the molecule. For 3-octyne, notice that the word "oct" signifies that the longest chain contains eight carbon atoms. As a first step (Step 1), we can draw out the oct- backbone. Next, we would look for any functional groups that we could add by looking either at the prefixes or the suffixes. In the case of 3-octyne, there is the suffix -yne, which signifies that there is a triple bond (alk yne ). The next step would be to identify where in the oct- backbone the alkyne appears. This info is usually given to us in the form of numbers, 3 in this case. So we can add in the triple bond in the 3- position. This is shown below in Step 2:
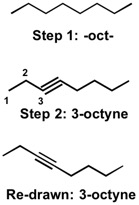
Note: Triple bonds are typically drawn as shown in the "re-drawn" structure, but the 3-octyne shown in Step 2 is also correct.

