What's the difference between a formula unit and a molecule?
1 Answer
A molecule is composed of two or more elements that are covalently bonded.
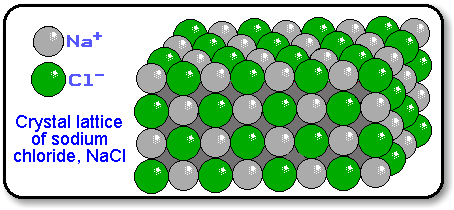
A formula unit indicates the lowest whole number ratio of ions in an ionic compound.
Explanation:
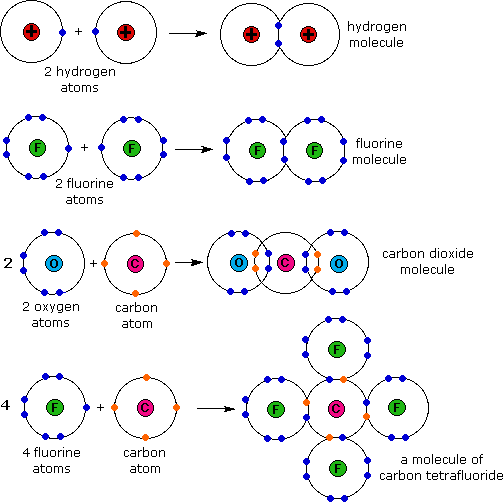
A molecule is composed of two or more elements that are covalently bonded. It is the smallest particle of a covalent substance that has all of the properties of that substance, and it is the smallest particle of that substance that can participate in a chemical reaction. The following diagram shows the formation of four different molecules through covalent bonding.
A formula unit indicates the lowest whole number ratio of ions in an ionic compound. Because the positively and negatively charged ions in an ionic compound are arranged in a crystal lattice, there is no discrete particle comparable to a molecule.