In order to determine the bond polarity between two atoms, their respective electronegativities must be determined. Electronegativity is the tendency of a bonded atom to attract bonded electrons to itself. The difference in electronegativity, #Delta"EN"#, can help predict the bond type.
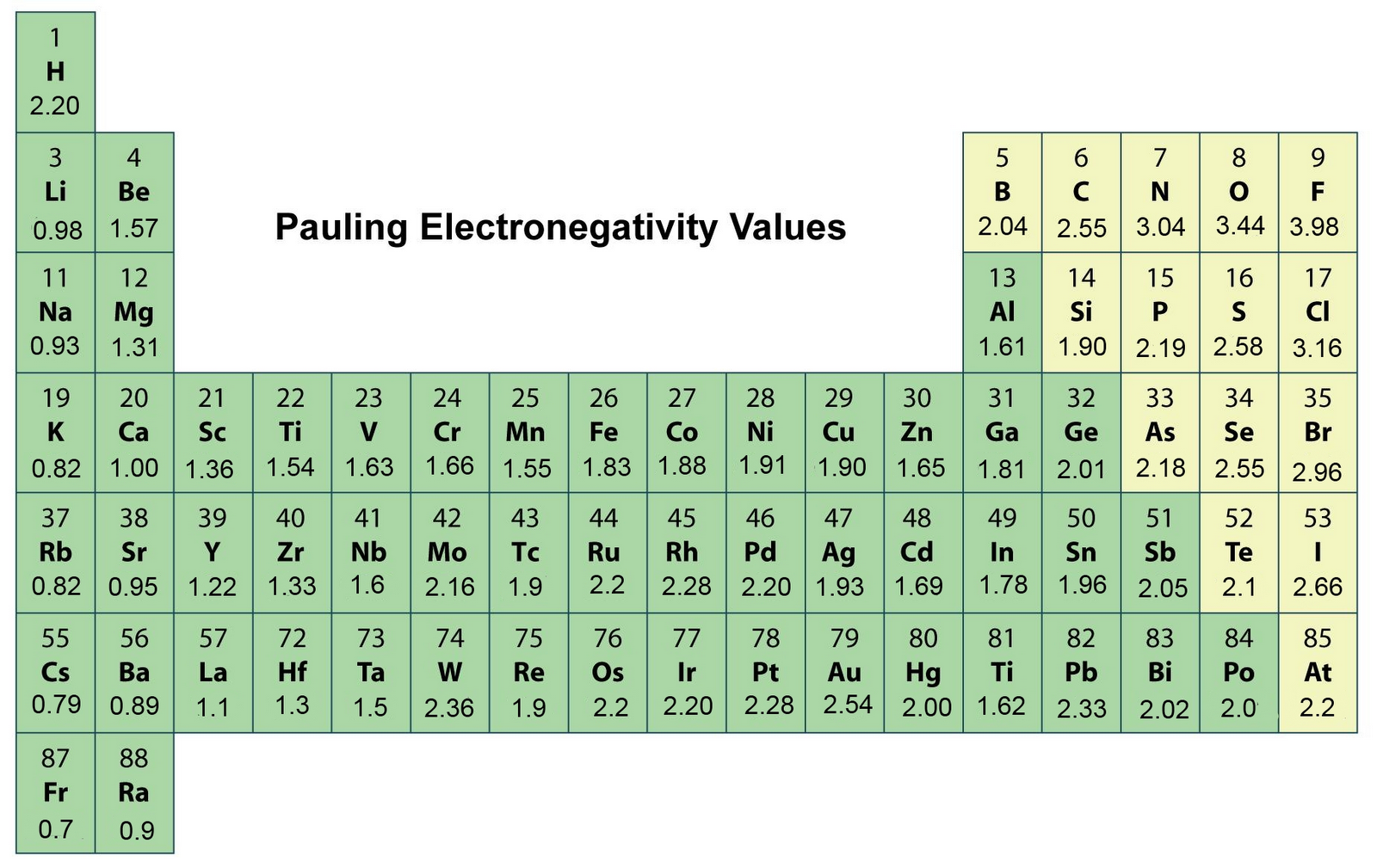
In general, a #Delta"EN"# of < 0.4 indicates a nonpolar bond, while a #Delta"EN"# of 0.4 - 1.7 indicates a polar covalent bond, and a #Delta"EN"# > 1.7 indicates an ionic bond. https://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text7/Tx71/tx71.html
For the C-H bond, the #Delta"EN"# = #"2.55 - 2.20 = 0.35"#. This is a nonpolar bond because a #Delta"EN"# of less than 0.4 is indicative of a nonpolar bond.
For the C-Cl bond, the #Delta"EN"# = #"|2.55 - 3.16|" = |0.61|"# (#Delta"EN"# is an absolute value; it is never negative.) The #Delta"EN"# of 0.61 indicates that the C-Cl bond is polar covalent.


