How can I explain the mechanism of acid-catalyzed epoxidation of alkenes with peroxy acids?
1 Answer
The reaction involves a cyclic concerted mechanism.
The Prilezhaev reaction is the reaction of an alkene with a peroxy acid such as m-chloroperoxybenzoic acid, MCPBA, to form an epoxide.
Electron-rich alkenes are more reactive.

The charge distribution in a peroxy acid puts a δ⁺ charge on the O atom of the OH group. This makes it an electrophilic atom that can add to alkenes.

The reaction proceeds via a concerted mechanism — all the steps happen at once.
We will describe them as if they were happening one after the other.
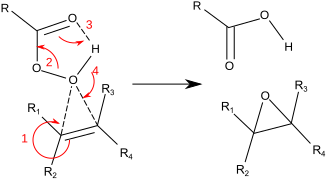
Step 1. The electrons in the π bond attack the electrophilic O atom. This forms a C-O bond and leaves a positive charge on the other C atom of the alkene.
Step 2. The O-O bond breaks, and the electrons move to form the new C=O group.
Step 3. The electrons from the old C=O group move to form an O-H bond with the H atom of the old OH group.
Step 4. The electrons from the old O-H bond move to form a C-O bond with the positively charged C atom of the alkene.
Here's a video on the epoxidation of alkenes by MCPBA.

