Epoxidation
Key Questions
-
Answer:
The reaction of alkenes with peroxides is often called an epoxidation reaction.
Explanation:
It is sometimes called the Prilezhaev reaction, after the Russian chemist who discovered it in 1909.
The reaction involves the action of a peroxyacid with an alkene to form an epoxide.

You can read more about this reaction in the Socratic answer to What is epoxidation of alkenes?.
-
Answer:
Well, you take an olefin, and make a three-membered ring...........
Explanation:
And from the three-membered epoxide ring, once ring-opened, you can get TWO functionalized sites.
And these days, epoxidation is not only highly chemically efficient and selective, it is increasingly highly stereochemically selective. Look up
#"Sharpless peroxidation"# , which won for its inventor, Barry Sharpless, a Nobel prize in 2001. -
All it means is that you are epoxidizing an asymmetric alkene with, for instance, a peroxyacid (this does not work with alkynes due to stability issues of the intermediate), as you have done early in your first semester of organic chemistry.
The concerted mechanism for the peroxyacid epoxidation of an asymmetric alkene is no different from that of a symmetric alkene other than the fact that the alkene looks different.
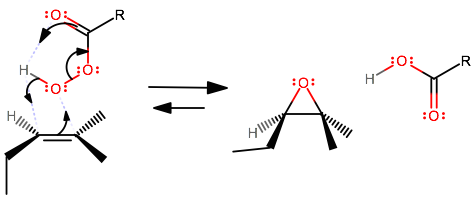
This epoxidation adds in a syn addition, and the enantiomers of this, where the epoxide oxygen is either in the back or in the front of the plane of the molecule, are both formed.
The syn addition is why the mechanism is the same for both symmetric and asymmetric alkenes.