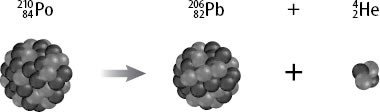
What is the nuclear equation for the alpha decay of Po210?
1 Answer
Jan 3, 2015
The nuclear equation that describes the alpha decay of Polonium-210 can be written like this:
Po-210 has 84 protons and 126 neutrons in its nucleus. During alpha decay, an
Since the Helium-4 nucleus has 2 protons and 2 neutrons, the alpha decay process will cause the atomic number to decrease by 2 and the atomic mass to decrease by 4.
Nuclear transmutation takes place after an aplha decay, which explains the formation of Pb-206.