What characteristic allows an atom to be detected by NMR?
1 Answer
NMR spectroscopy depends on the quantized magnetic properties of a nucleus.
Protons and neutrons behave as if they are spinning on their axes.
If you have equal numbers of protons and neutrons, as in
If the nucleus has an odd number of nucleons, as in
A nucleus with
These orientations have the same energy in the absence of a magnetic field.
In the presence of a magnetic field, these energy levels split. Each level is labelled with a magnetic quantum number
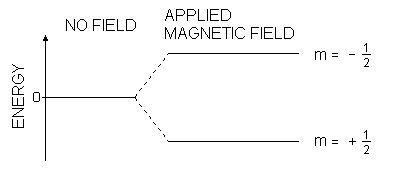
Levels with

Suppose you irradiate the sample with electromagnetic radiation of the correct frequency so that
The irradiation can induce a nucleus in the low energy state to absorb energy and "jump" to the high energy state.
Sensitive instruments can detect this absorption of energy.
This is how NMR spectroscopy can detect an atom.

