What type of bonding is in the #sf(N_2)# molecule ?
1 Answer
Jan 7, 2015
In the nitrogen molecule the atoms are held together by triple covalent bonds.
The electron structure of nitrogen is
There are therefore 5 valence electrons. These are able to form a triple bond with one non - bonding pair on each atom:

The molecular orbital diagram is:
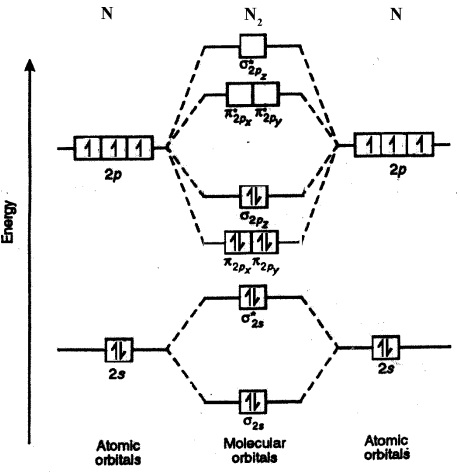
The
The

Here we can see that the number of bonding electrons (
The bond order

