If the ring closure is an S_N2 reaction then what should be the arrangement of the -O-C-C-X system?
1 Answer
Jan 9, 2015
The O-C and C-X bonds must be anti to each other.
One way to prepare an epoxide is to react a halohydrin with a base.
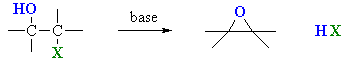 www.chem.ucalgary.ca
www.chem.ucalgary.ca
An example is the conversion of 3-bromo-2,3-dimethylbutan-2-ol to 2,3-epoxy-2,3-dimethylbutane.
 wikipremed.com
wikipremed.com
The reaction is really an intramolecular Williamson ether synthesis.
Step 1 is an acid-base reaction. The OH⁻ removes a proton from the alcohol. This converts it to an alkoxide ion and enhances the nucleophilicity of the oxygen atom.
Step 2 is an intramolecular
The C-O⁻ bond and the C-Br bond must therefore be anti to each other.

