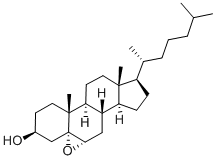
Why is the epoxidation of cholesterol stereoselective?
1 Answer
Jan 19, 2015
The epoxidation of cholesterol is stereoselective because the C-19 methyl group hinders attack from the top side of the molecule.
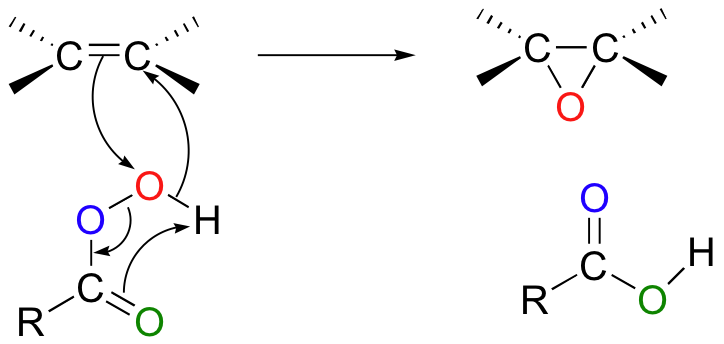
Epoxidation involves the concerted syn attack of a peroxy acid on an alkene.
The structure of cholesterol is
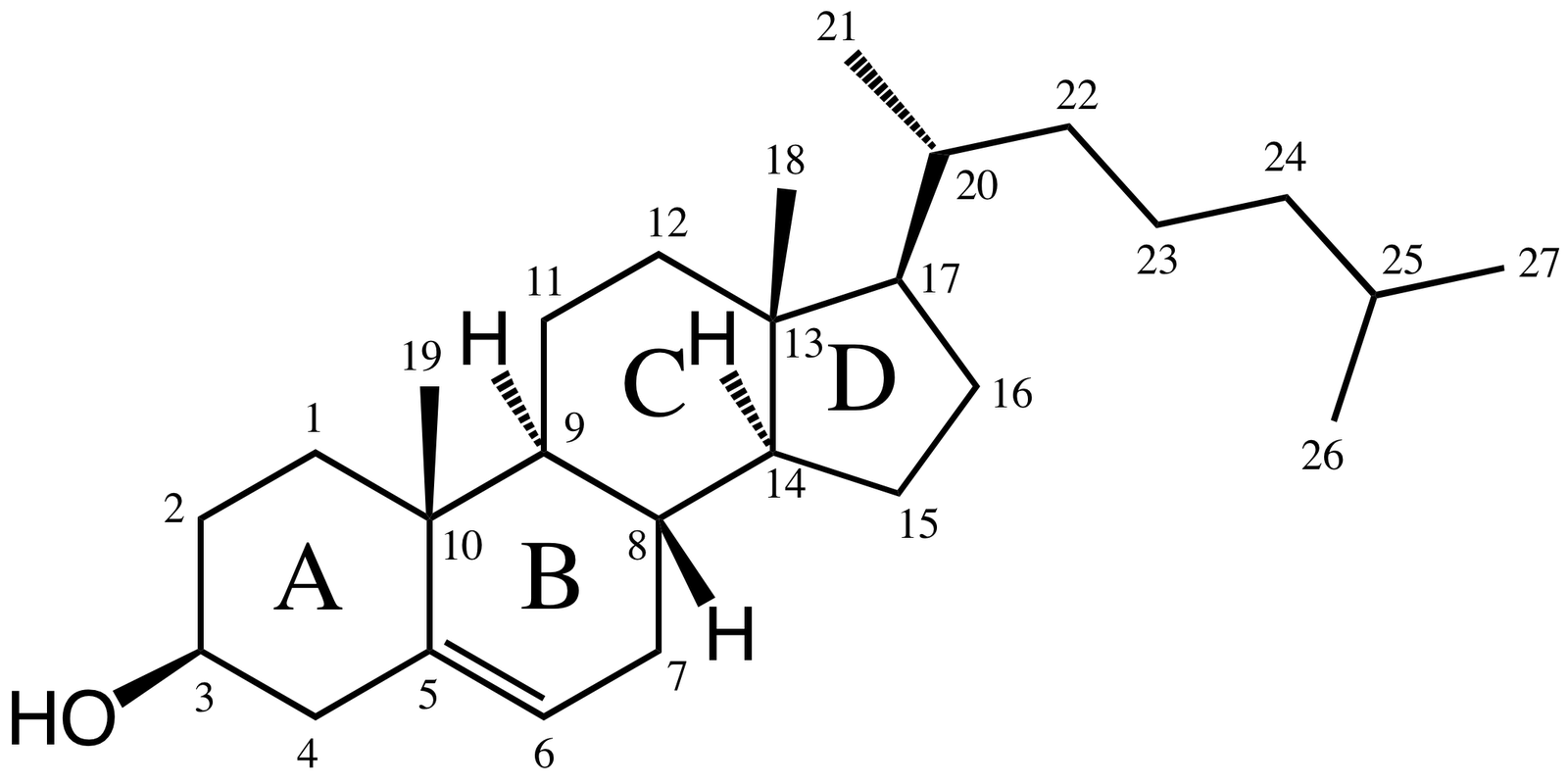
There are eight chiral centres. The one of concern to us is C-10 and the methyl group (C-19) attached to it.
The CH₃ group is "up". It is bulky enough to provide much steric hindrance to a reactant trying to attack C-5 and C-6 from the top side.
Hence the epoxide forms exclusively on the unhindered "bottom" side of the molecule.
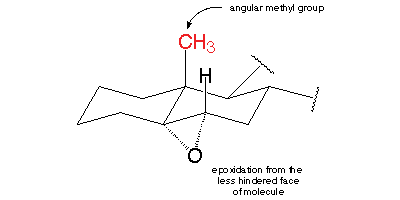
The only significant product is 5α,6α-epoxycholestan-3β-ol (α is "down"; β is "up").