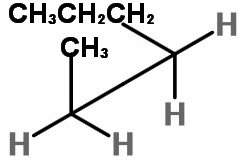
How can I draw Sawhorse projections for the #C_2-C_3# bond of hexane?
1 Answer
You can find the general procedure for drawing sawhorse projections at this location.
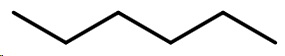
The condensed structural formula of hexane is CH₃-CH₂CH₂-CH₂CH₂CH₃.
Its bond-line structure is
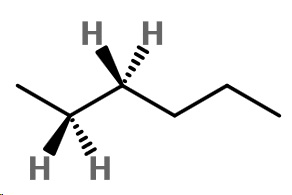
Step 1. Convert the bond-line structure to a wedge-dash structure at C-2 and C-3.
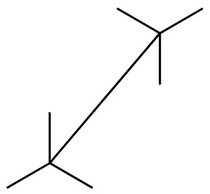
Step 2. Convert the wedge-dash structure to a sawhorse projection.
We must look at the molecule along the C2-C3 bond (from the lower left).
The three bonds nearest us form an inverted "Y".
We draw a sawhorse template with the C2-C3 bond going from lower left to upper right.
Next we add the atoms.
The groups on C-2 are CH₃, H, and H.
The groups on C-3 are CH₃CH₂CH₂, H, and H.
On C-2, we put the bulky CH₃ group at the top of the inverted "Y",
On C-3, we put the bulky CH₃CH₂CH₂ group at the bottom of the "Y", with the H atoms on the other two bonds.
This staggered conformation puts the two bulky groups anti to each other and is the most stable form.
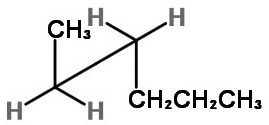
To get the two gauche forms, hold the front carbon in in place and rotate the back carbon in 120° increments. You get an enantiomeric pair of rotational isomers:
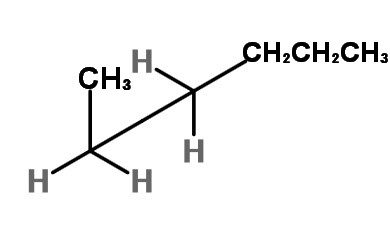
and