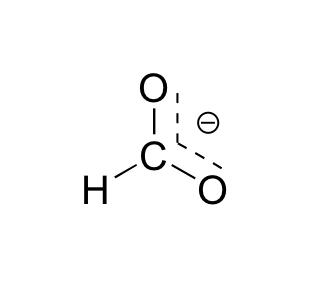
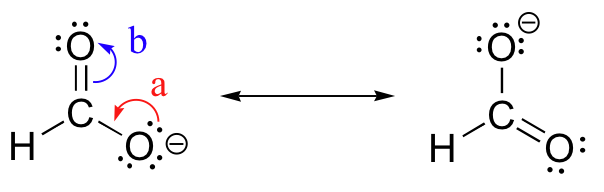
What are the major and minor resonance contributor(s) for the formate anion, #HCO_2^–#?
1 Answer
The formate anion, or
Here are the three resonance structures for the formate anion
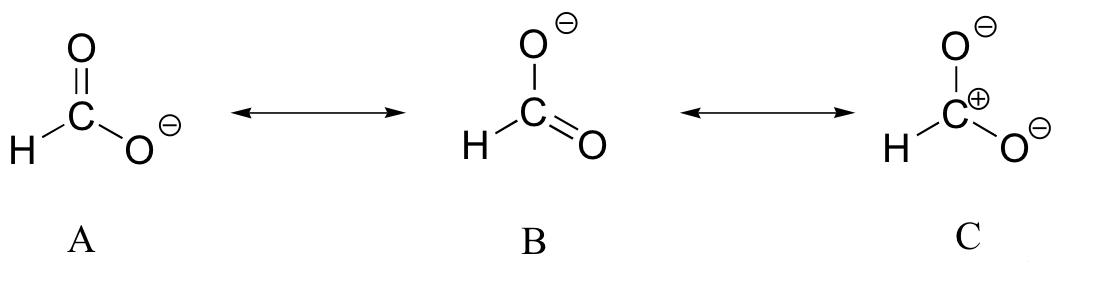
Let's analyze all these three resonance structures in order to determine the minor and major contributors.
Structure A and structure B are equivalent from the stability standpoint; both structures have full octets for all the atoms involved, and the negative charge is placed on the electronegative atom, oxygen.
Structure C is the odd one out because it practically lacks all the characteristics of a major resonance structure. The most important difference between C and the other two structures is the fact that the carbon atom has an incomplete octet.
Next in line is the number of covalent bonds. The greater the number of covalent bonds a resonance has, the more stable and the more important it is. A and B each have 4 covalent bonds, while C has only 3.
Moving on to separation of charge. Stable resonance structures have the least amount of charge separation, which automatically implies, in this case, that C is not as stable as A and B.
Therefore, the formate anion has 2 major resonance structures, labeled here A and B, and one minor contributor, structure C. Here's how the hybrid structure for the formate ion would look like