What is the mechanism of an aldol addition?
1 Answer
Here's what I find.
Explanation:
An aldol addition is the addition of an enolate of an aldehyde or a ketone to the carbonyl carbon of another molecule under basic or acidic conditions to obtain a β-hydroxyaldehyde or β-hydroxyketone (an aldol).
An example of aldol addition is the base-catalyzed reaction of acetaldehyde with itself to form 3-hydroxybutanal.
There are three steps in the aldol addition.
1. Removal of an acidic α-hydrogen by a strong base to form a resonance-stabilized enolate ion.
 www.organic-chemistry.org
www.organic-chemistry.org
2. Nucleophilic attack of the enolate ion on the electrophilic C=O group of another molecule forms a new C-C bond and an alkoxide ion.
3. Protonation of the alkoxide ion to form the aldol.
A condensation is a reaction in which two molecules combine to form a larger molecule together with the loss of a small molecule such as water.
If the aldol is then dehydrated, the overall process is called an aldol condensation.
Heating the aldol with strong base results in the elimination of water and the formation of an α,β-unsaturated product.
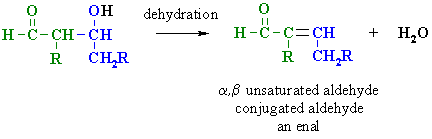 www.chem.ucalgary.ca
www.chem.ucalgary.ca
The video below gives a good overview of aldol addition and condensation.

