Halogenation
Key Questions
-
It's the addition of a halogen to the C=C double bond of an alkene.
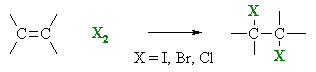
An example is the addition of chlorine to ethylene.

The product has Cl atoms on adjacent carbon atoms.
It's the addition of a halogen to the C=C double bond of an alkene.
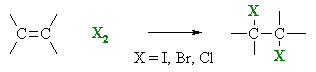
An example is the addition of chlorine to ethylene.

The product has Cl atoms on adjacent carbon atoms.