Question #f08d8
1 Answer
PMMA doesn't dissolve in water because the London dispersion forces among adjacent molecules prevent the molecules from going into solution.
PMMA is a polymer of methyl methacrylate.
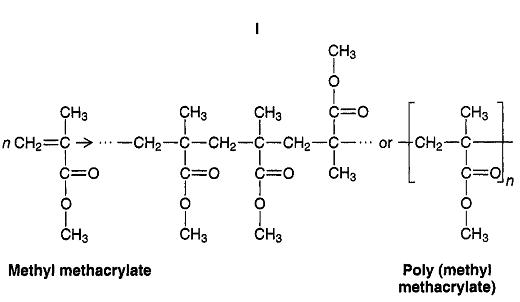
It is a long hydrocarbon chain with CH₃ and COOCH₃ groups hanging off the side.
A hydrocarbon chain is nonpolar. So is the CH₃ side chain. So that part of the molecule is not attracted to a water molecule.
The COOCH₃ groups are slightly polar, because of the polar C=O and C-O bonds. They would be attracted to water molecules.
But the small attraction is offset by the hydrophobic nature of the main chain and the methyl groups.
The main factor is the London dispersion forces among adjacent polymer chains.
The attractions between adjacent chains is huge, because the molecules can line up side-by-side against each other for long distances. They are the most important force in the polymer.
Individual chains cannot separate from the polymer molecule and go into solution, so PMMA is insoluble in water.

