Question #90f72
2 Answers
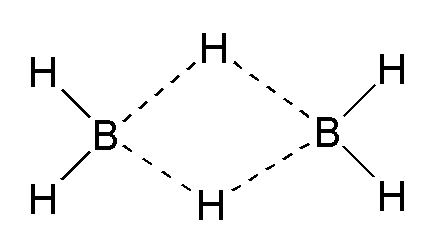
Yes, diborane, or
Diborane has more bonding orbitals than it has valence electrons, which makes it electron deficient. The molecule has a total of 12 valence electrons - 3 from each boron atom and 1 from each hydrogen atom. However, it only has 10 bonding orbitals - 4 from each boron atom and 6 from each hydrogen atom.
Excited state boron is
These bonds will use up 8 of the 12 valence electrons. The other 4 valence electrons will be shared by the remaining two hydrogen atoms and by the boron atoms , each atom contributing one orbital to the formation of the 3-center 2-electron bond.
This is what a three-center two-electron bond actually is - three atoms sharing two electrons.
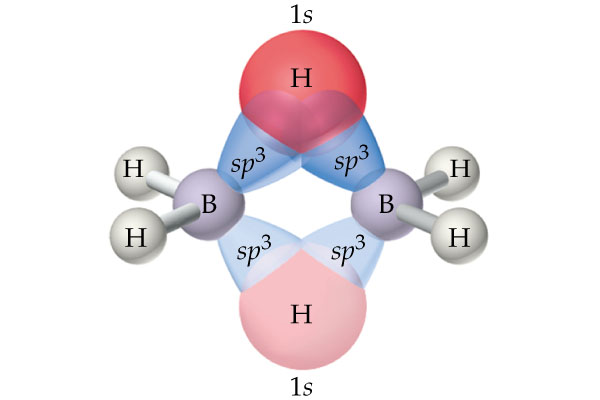
As you can see, each boron uses two of its four
The strong electrostatic repulsion felt by the positively charged nuclei of the two hydrogen atoms that form the hydrogen bridge (due to the proximity of the atoms) will cause the bond to be bent - you'll sometimes see this referred to as a banana bond.
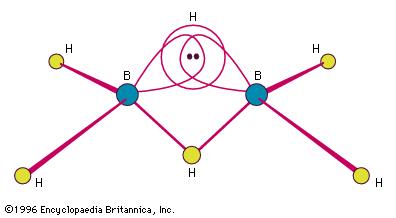
Here's a more exact image of what makes up the 3-c 2-electrron bond
Each of the two hydrogen bridges will contain 1 electron from a boron atom and 1 electron from a hydrogen atom, with three orbitals coming together to form the bridge.
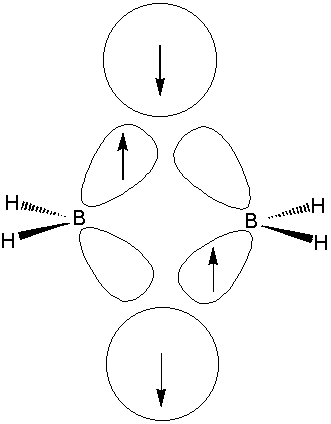
In diborane
The terminal B - H bonds may be regarded as conventional 2 centre - 2 electron bonds. Each boron atom uses 2 electrons and and 2 roughly
This means the boron atoms still have one electron and 2
Although the graphic I have posted from Wiki implies a planar molecule, the plane of the BHB bonds is perpendicular to the plane of your computer screen.
Since we have only 2 electrons spread over 3 centres the molecule can be considered to be "electron deficient".
The 3 atomic orbitals combine to give 3 linear combinations of molecular orbitals:



