Which following pairs of atoms, have a lower electron affinity? a) Ca,K b) I,F c) Li, Ra. I seriously don't know anything about electron affinity all ik that it can buy another element
3 Answers
Electron affinity (EA) expresses how much energy is released when a neutral atom in the gaseous state gains an electron from an anion.
Periodic trends in electron affinity are as follows: electron affinity (EA) increases from left to right across a period (row) and decreases from top to bottom across a group (column).
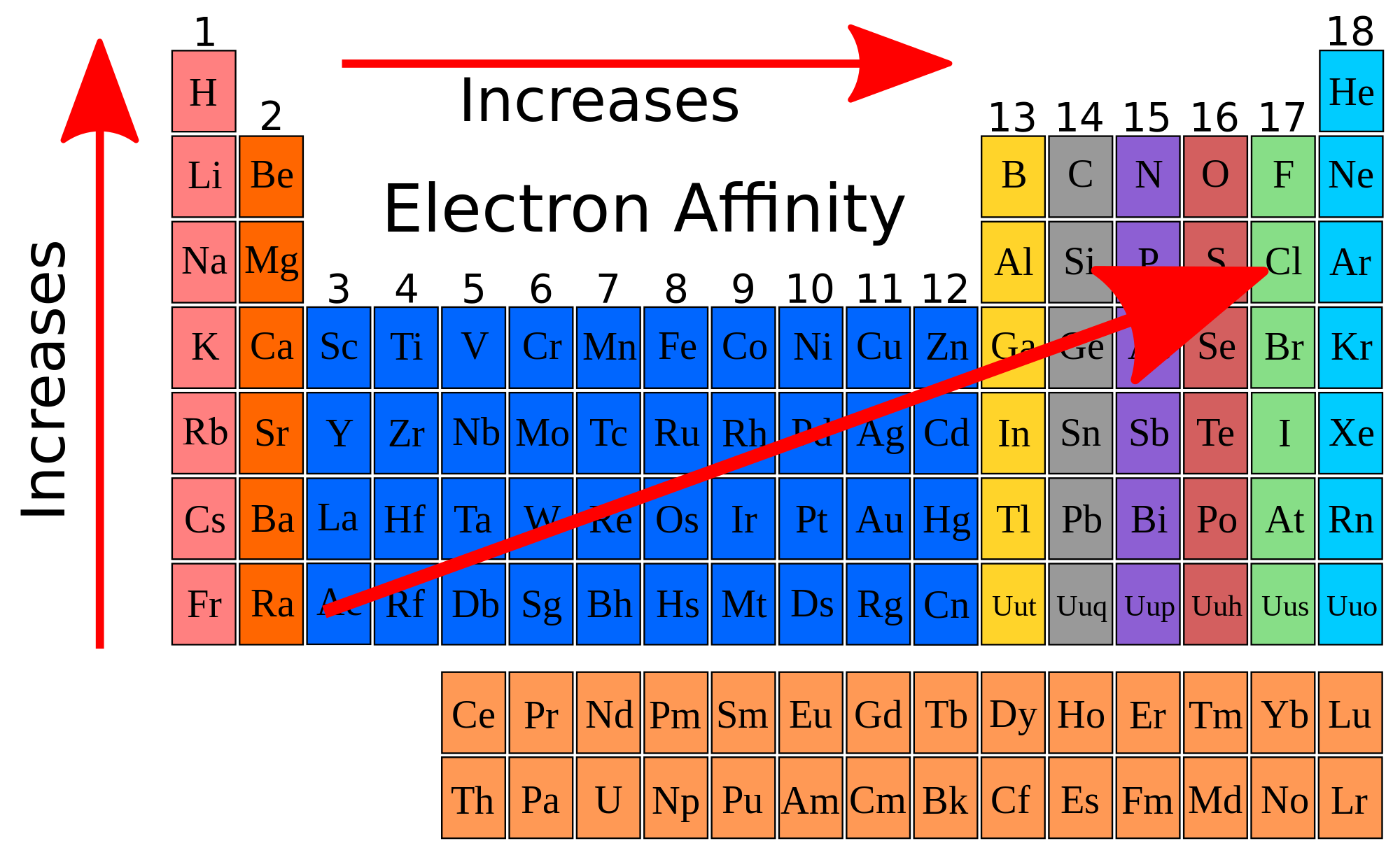
So, when you have to compare two elements that are in the same period, the one further to the right will have a greater EA. For two elements in the same group, the one closest to the top will have a greater EA.
Since
For
A magnesium atom has the larger radius.
A magnesium atom has a radius of 150pm.
A magnesium ion has a radius of 72pm.
The electron structure of a magnesium atom is
The 2 outer electrons are lost to form a
This accounts for the decrease in radius.
Electron affinity is the ability to attract an electron into the outer shell of an atom, either to become an 1- ion, or to become a more negatively charged ion e.g. 2- etc.
The ability to do this increases with the nuclear charge (i.e. with more protons in the nucleus) and with fewer filled inner shells causing shielding. N.B. These two factors also result in smaller radius, so smaller radius will correspond to higher electron affinity.
This means that electron affinity increases as we go left-to-right across a period (same number of shells, more protons) and decreases as we go down a group (more shells and larger radius outweigh the increasing number of protons).
So:
a) K will have a lower electron affinity that Ca
b) I will have a lower electron affinity than F
c) Ra will have a lower electron affinity than Li
Since electron affinities relate to the formation of negative ions, however, it is unusual to consider them for metals. I has an electron affinity of -295kJ/mol compared to -328kJ/mol for F, so F has a more exothermic first electron affinity.
Source: Nuffield Advance Science Book of Data




