Question #ebbe0
1 Answer
The mass of the collected hydrogen gas is
Once again, here's the reaction wou're working with
The basic idea behind this reaction is that the hydrogen gas will be collected over water at a total pressure that includes the water vapor.
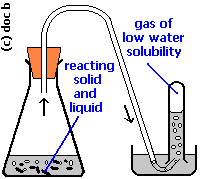
So, in order to determine the pressure of the hydrogen gas, you must remove the water vapor from the total pressure
In this case,
Now just use the ideal gas law equation to solve for the moles of hydrogen produced
Once again, use the units required for this value of R - atm, L, and K!
Now just use hydrogen's molar mass to determine the actual mass

