What do these sets of quantum numbers describe?
#a)# #(n,l,m_l,m_s) = (5,1,-1,+1/2)#
#b)# #(n,l,m_l,m_s) = (6,1,1,-1/2)#
1 Answer
As I'm sure you know, there are four quantum numbers used to describe the location of an electron.
In order, these four quantum numbers are
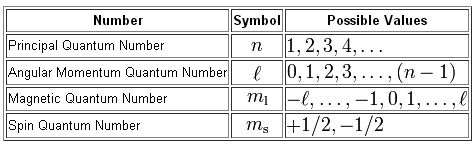
I'll start with the first one. The 5p subshell places your electron in one of the three 5p-orbitals,
This set describes an electron that is located on the fifth energy level, in the 5p subshell, in the
Keep in mind, n = 5 and l = 1 are a must if you want your electron to be in the 5p subshell. You could however have other values for
Now for the second one. The 6p subshell has, once again, three 6p-orbitals:
This time, the numbers I've chosen describe an electron that is located on the sixth energy level, in the 6p subshell, in the

