Question #d7af1
1 Answer
Atomic size decreases when moving from left to right across a period, or a row, of the periodic table. This periodic trend in atomic size is attributed to the increasing effective nuclear charge, or
Now,
Across a period, all the electrons added to the atom are placed on the same energy level, i.e. at the same distance from the nucleus. This will have an impact on atomic size because it influences the magnitude of the attraction force the electrons feel from the nucleus.
In essence, elements that are in the same period have the same number of core electrons, which implies that all of the added electrons will experience the same amount of screening from the nucleus.
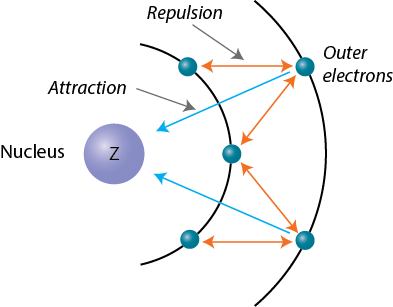
However, as the attraction from the nucleus increases, i.e the number of protons increases by 1 across a period, the screening effect the core electrons provide to the valence electrons will become less efficient.
As a result, the pull from the nucleus will be stronger with every added proton, so the electrons will be held tighter
Here's how that would look for the elements in period 3
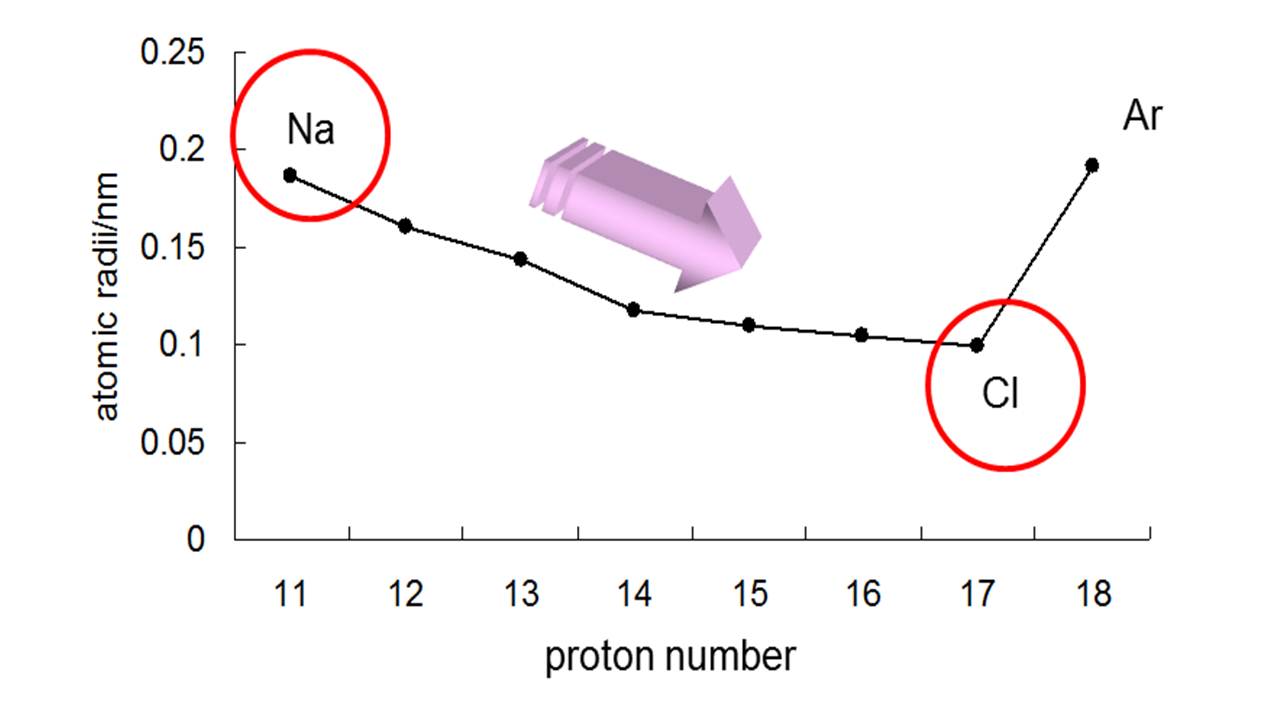
Notice that
I won't go into detail, but you should ignore noble gases because their measured radius is actually the van der Waals radius, which is not the same thing as the atomic radii of the other elements.

