Question #e9926
2 Answers
It is d. the number of protons in the nucleus that makes an oxygen atom different from a carbon atom.
The atomic number Z, the number of protons in the nucleus, determines the identity of an element.
For carbon, Z = 6. For oxygen, O = 8.
This is how you decide which element is which.
The answer would be d) the number of protons.
Elements can and do differ in the number of neutrons, electrons, and valence electrons, but what makes an element unique is the number of protons in has in its nucleus.
The number of protons an element has in its nucleus is known as the atomic number and, like I've said, is unique to each atom.
Carbon is in period 2, group 14 of the periodic table, and has an atomic number equal to 6. This means that a neutral carbon atom has 6 protons in its nucleus and 6 electrons surrounding the nucleus.
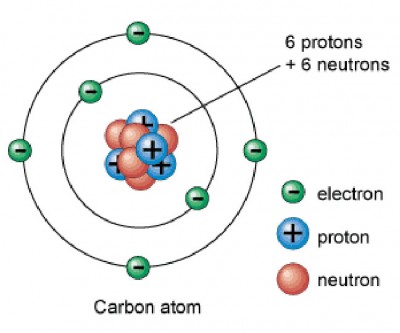
Oxygen, on the other hand, is located in period 2, group 16 of the periodic table, which means it has and atomic number equal to 8 - 8 protons in its nucleus and 8 electrons surrounding the nucleus.
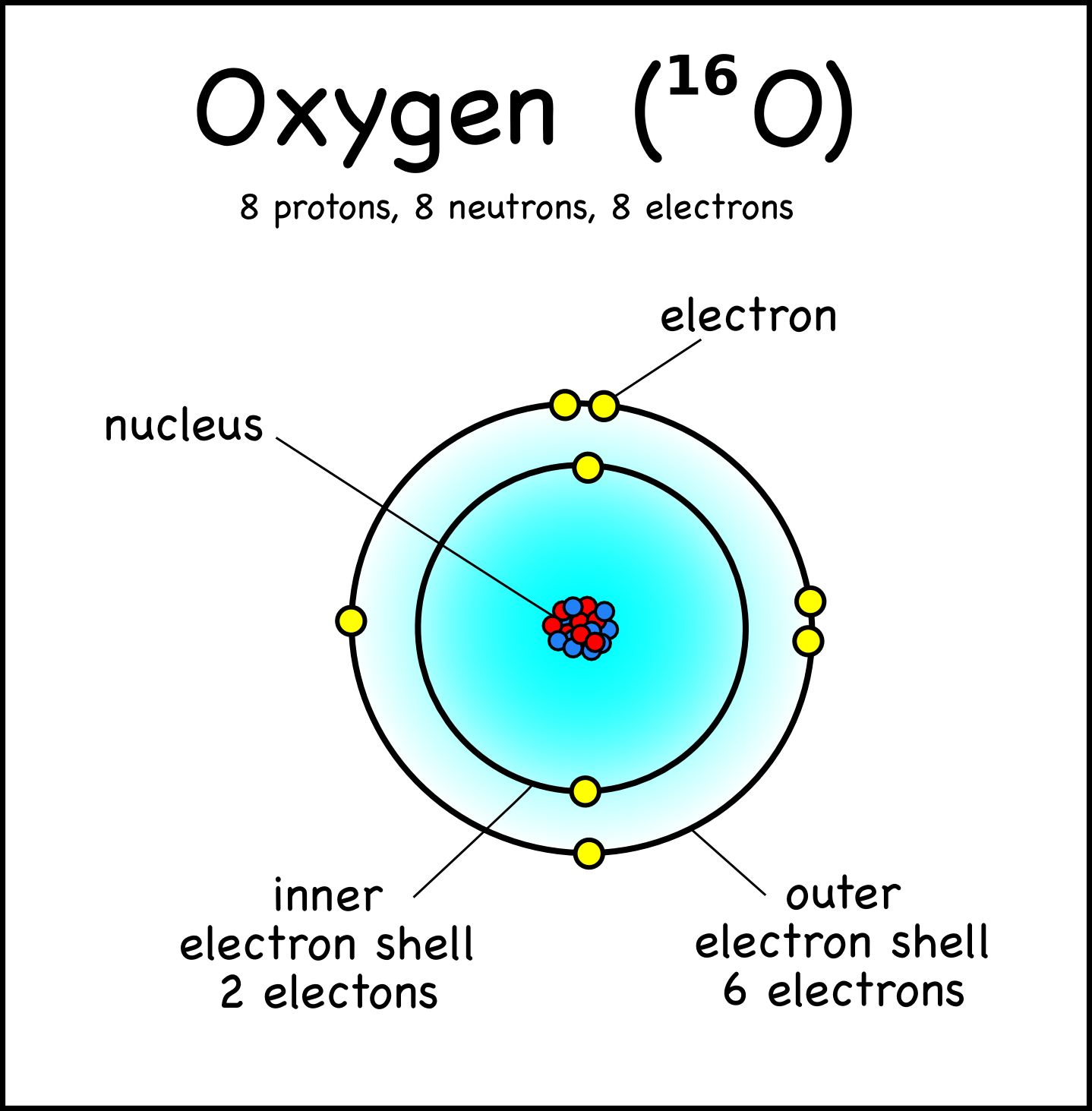
So, as a conclusion, you can never have two distinct elements with the same atomic number, i.e the same number of protons in the nucleus.


