Which is more important in determining if a molecule is polar, symmetry or the difference of electronegativity? For example, CF4 is tetrahedral so it's symmetrical but C and F have a difference of 1.5 in electronegativity, which is more than .4
1 Answer
Both the symmetry of the molecule and the polarity of the bonds are equally important in determining the polarity of a molecule.
These two factors are not competing against each other in any way, so one cannot say that symmetry is more important than polarity of bonds, or vice versa.
If you don't have polar bonds, you can't have a polar molecule regardless of the symmetry. If you do have polar bonds, you can't have a polar molecule unless you have a favorable molecular geometry.
You can't have one without the other and have a polar molecule. A polar molecules requires the presence of a permanent dipole moment, which in turn cannot come about without polar bonds and favorable geometry.
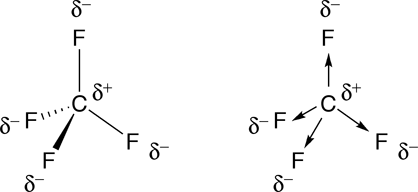
As you know, in the case of
As a conclusion, the polarity of a molecule depends on both molecular geometry and polarity of bonds. If either one of these two factors is not favorable, you won't have a polar molecule.
Here is a video to help with this concept.
video from: Noel Pauller


