What is the name for #P_2O_5#?
3 Answers
The name is diphosphorus pentoxide.
Binary compounds with oxygen in them have 'oxide' as a 'last name'.
The 'first name' is phosphorus.
We give the numbers of each atom:
di (=2) phosphorus penta (=5) oxide, which means: 2 phosphorus together with 5 oxygen
The 'a' of 'penta' is omitted when it comes before 'o' as in 'oxide'.
Often the 'di-' is also omitted (as the other compound also starts with 'di-'), and it is called just phosphorus pentoxide.
Extra :
An older name would be phosphoric oxide,
The systematic name for
In the same way
An interesting fact about phosphorus pentoxide is that
The compound's name however was derived from its empirical formula, not from its molecular formula. The standard name for this compound is actually diphosphorus pentoxide.
The di- prefix is used to show that the compound contains two phosphorus atoms and the penta- prefix is used to show it contains five oxygen atoms.
Phosphorus pentoxide is the most used name for
This is the image of the molecule from wikipedia.
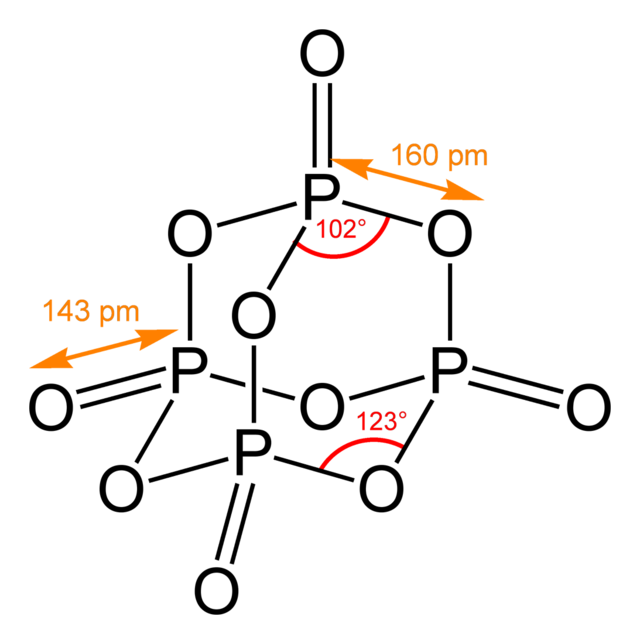
For its avidity of water, it is one of the most powerful known dehydrating agent.




