Question #e4180
2 Answers
You do the basic steps as you would for any molecule.
Start by drawing the Lewis structure of the
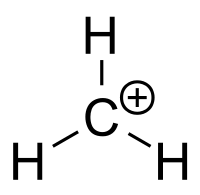
To determine hybridization, count all the electron-dense regions, i.e covalent bonds or lone pairs, that are located around the central atom - this is know as the steric number.
Notice that the carbon atom bonded to three hydrogen atoms, which implies that its steric number is equal to 3.

This means that the central atom is
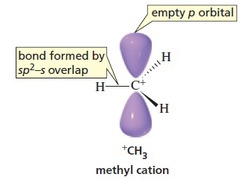
The geometry of the molecule will be trigonal planar with
Methylium cation,
The leftover


