Question #7f0a7
1 Answer
Apr 8, 2015
Phosphoric acid, or
The Lewis structure of phosphoric acid looks like this:
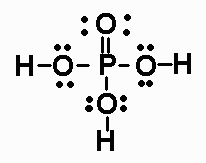
The phosphorus atom has zero lone pairs, and uses all its five valence electrons to form bonds with the four oxygen atoms - 3 single bonds and 1 double bond.
As you can see, the five bonds it forms give phosphorus a total of 10 electrons in its valence shell, which implies that it does not obey the octet rule.
However, because phosphorus is located in period 3 of the periodic table, it can use its d-orbitals to accomodate more than 8 electrons in its valence shell.

