Which of the following is not a valid set of quantum numbers? A) n = 3, l = 0, ml = 0, and ms = 1/2 B) n = 2, l = 1, ml = -1, and ms = -1/2 C) n = 3, l = 2, ml = 3, and ms = 1/2 D) n = 2, l = 1, ml = 0, and ms = -1/2
1 Answer
Apr 16, 2015
The answer is C).
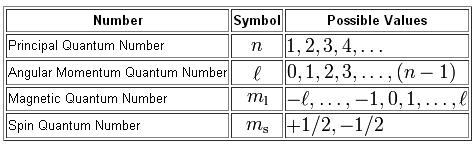
The magnetic quantum number, or
For option C), the angular momentum quantum number of equal to ++2, which means that
The other three sets are valid and can correctly describe an electron.

