what type of solution will be created when 35 grams of Nh4Cl is added to 25 ml of water at 80 Celsius?
1 Answer
You will create a saturated solution.
When you add more solute than a solvent can dissolve at a specific temperature, you'll create a saturated solution. The solute will dissolve in the limit of its solubility; anything that exceeds that amount will remain undissolved in solution.
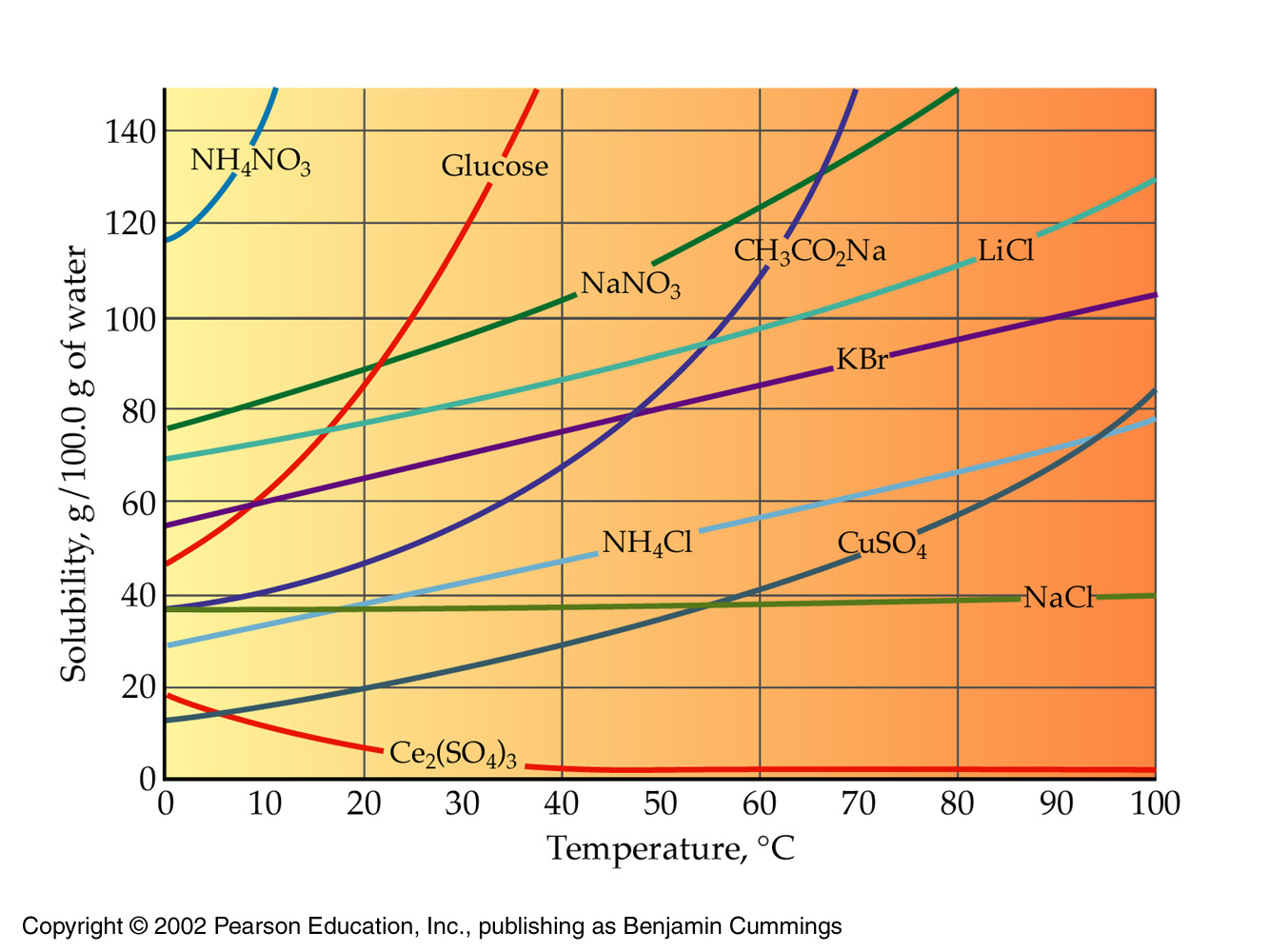
The solubility of ammonium chloride is listed at 65.6 per 100.0 g of water at
At that temperature, the density of water is 0.9718 g/mL (http://antoine.frostburg.edu/chem/senese/javascript/water-density.html), which means that you'll get
This much water can dissolve
Since you've added 35 g of ammonium chloride to the solution, you've created a saturated solution. Your solution will contain an undissolved mass of

