Question #f6b1f
1 Answer
The correct order will be
Because only three of the four elements listed are in the same period of the periodic table, you'll have to take into account both periodic trends in electronegativity.
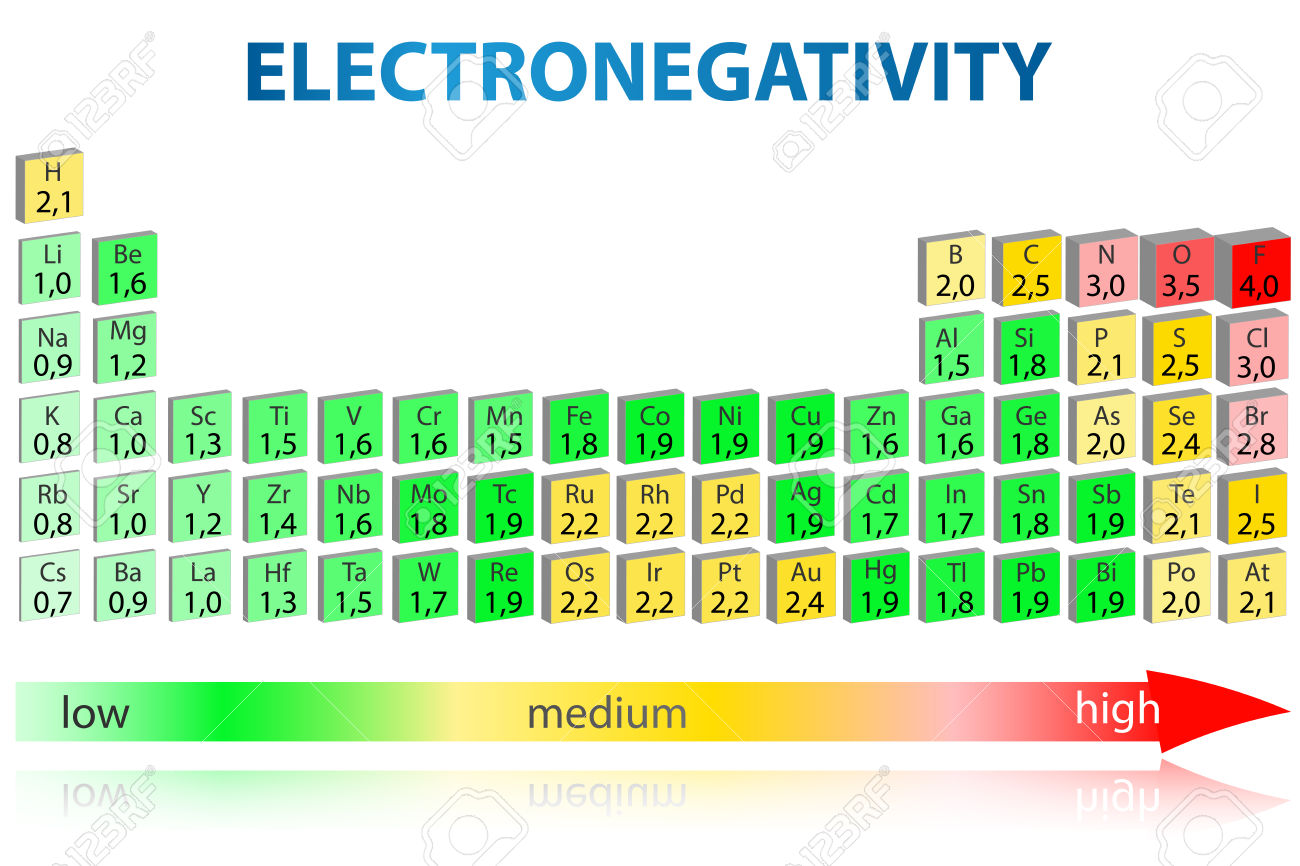
So, electronegativity (EN) increases when going from left to right across a period, and decreases when going down a group.
RIght off the bat, oxygen will be the most electronegative of the group because it's located in period 2, group 16, as opposed to the other three elements which are located in various groups in period 3.
For the other three elements, sodium will be the least electronegative of the group, since it's located in group 1, and sulfur will be the most electronegative, since it's located in group 16.
As a result, in order of increasing electronegativity, you'll get

