How does a catalyst make Hydrogen Peroxide's decomposition quicker? What is actually happening?
1 Answer
A catalyst makes the decompostition reaction of hydrogen peroxide faster because it provides an alternative pathway with a lower activation energy for the reaction to take.
Activation energy is just a term used to express the minimum energy required in order for a reaction to take place.
If no catalyst is present, hydrogen peroxide will decompose at a very, very slow rate - I think its concentration will drop by 10% per year.
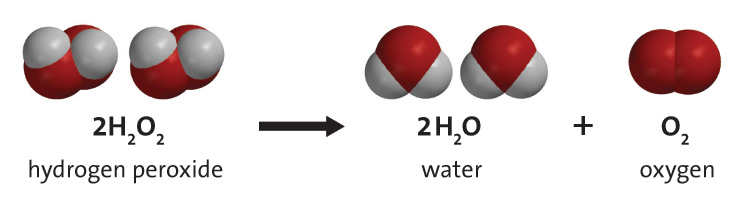
When a catalyst is added, an alternative pathway through which the reaction can form water and oxygen gas is introduced. The speed of a catalyzed reaction will increase because this alternative pathway has a lower activation energy.
Here's what an alternative pathway means. For example, let's say you add potassium iodide,
Potassium iodide will dissociate completely to give potassium ions,
An iodide ion will react with a hydrogen peroxide to produce water and a hypoiodite ion,
That's why a catalyst is never consumed in a reaction - it is reformed at the end of the multi-step reaction.
Adding equations
In this case, the activation energy for the potassium iodide-catalyzed reaction is
Lower activation energy


