Question #389db
1 Answer
Jun 6, 2015
Under those conditions for pressure and temperature, water can only exist as liquid.
Take a look at water's phase diagram
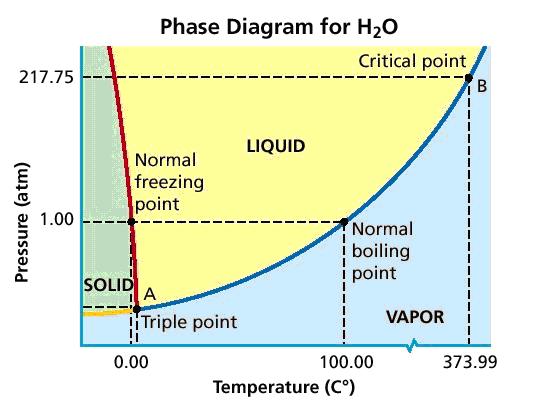
Normal atmospheric pressure and room temperature correspond to a pressure of 1 atm and a temperature of
Notice that, at 1 atm, water can exist as a solid only at temperatures below
At this pressure and at any temperature higher then

