What is the structure of the molecule with the name (E)-3-benzyl-2,5-dichloro-4-methyl-3-hexene?
1 Answer
Jun 9, 2015
The structure of (E)-3-benzyl-2,5-dichloro-4-methylhex -3-ene is
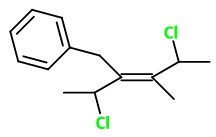
Explanation:
The real problem here is determining the (E,Z) configuration of the molecule.
You draw a hexane chain with the double bond between
Determine the priorities of the groups on
- Benzyl. The atoms directly attached to the methylene carbon atom are (
#"C,H,H"# ). - 1-Chloroethyl. The atoms directly attached to
#"C-1"# are (#"Cl,C,H"# ).
1-Chloroethyl has higher priority than benzyl because
Determine the priorities of the groups on
- 1-Chloroethyl. The atoms directly attached to
#"C-1"# are (#"Cl,C,H"# ). - Methyl. The atoms directly attached to the methyl carbon atom are (
#"H,H,H"# ).
1-Chloroethyl has higher priority than methyl because#color(lime)"Cl"# has a higher atomic number than#"H"# .
In each case, the 1-chloroethyl group has the higher priority.
These groups must therefore be on opposite sides of the double bond (entgegen).

