Question #8dcb5
1 Answer
Because very little to no interaction exists between molecules in the gas phase.
Explanation:
The strength of the intermolecular forces of attraction is much greater in liquids than it is in gases.
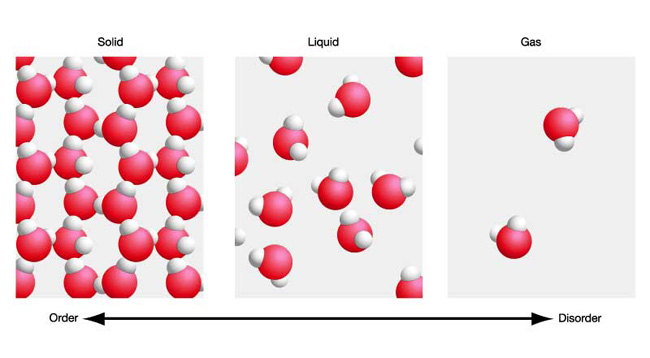
One gram of liquid water and one gram of steam contain the same number of molecules. However, the molecules in liquid water are still very much in contact with each other.
The intermolecular forces that exist between water molecules, the most important one to note being hydrogen bonds, keep these molecules from moving about freely.
This is why liquid water has a fixed volume.
When you provide enough energy to go from liquid water to steam, you break the intermolecular bonds that keep the molecules close to each other.
As a result, the molecules are now free to move around almost independently of one another. Since no more attractions exist between the molecules, they can move about in any direction, which is why the volume will no longer be fixed.

