How can I draw d -glucose in its chair conformation? Why it is the most common aldohexose in nature?
1 Answer
First convert the Fischer projection to a Haworth projection, then convert the Haworth projection to a chair form.
Explanation:
The Fischer projection of glucose is

Convert to a Haworth Projection
Step 1. Draw a basic Haworth projection with the ring oxygen at the top.
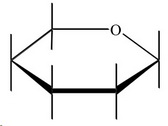
Step 2. Draw a
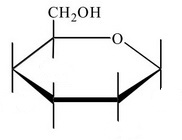
Step 3. Draw an
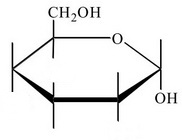
Step 4. Draw all the
The
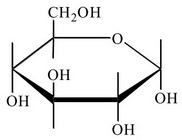
You can omit the hydrogen atoms, so the Haworth projection for α-D-glucopyranose is

Convert Haworth to Chair
Step 1. Draw a cyclohexane chair in which the

Step 2. Put all the
The chair form of α-D-glucopyranose is

The structure of β-D-glucopyranose is

Prevalence of Glucose
As you move around the β-glucose ring, you see that all the substituents are equatorial.
This is the most stable arrangement possible.
In α-glucose, only the
Every other aldohexose would have more axial substituents and be less stable.
Glucose is the most common hexose because it is the most stable.

