The molecule #SO_2# would be expected to have ? (A) two identical bonds intermediate between a single and a double bond (B) two single bonds (C) two double bonds (D) one single and one double bond
1 Answer
The answer is (A) two identical bonds intermediate between a single and a double bond.
Explanation:
In order to be able to predict what type of bonds the sulfur dioxide molecule will have, you need to figure out what its Lewis structure looks like.
Sulfur dioxide has a total of 18 valence electrons: 6 from the sulfur atom and 6 from each of the two oxygen atoms.
One way of drawing the molecule's Lewis structure has the sulfur atom bonded to the two oxygen atoms vi double bonds, with two lone pairs of electrons present on each of the oxygens.
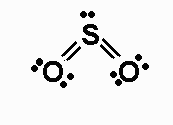
The remaining 2 valence electrons are placed on the sulfur atom as a lone pair.
If you break one of the two double bonds and move two bonding electrons on an oxygen atom, you can draw two additional Lewis structures for sulfur dioxide.
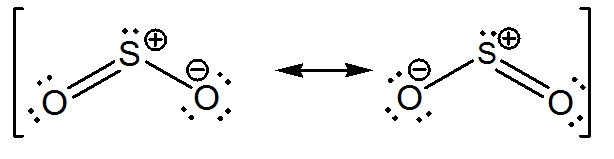
These structures are called resonance structures and they contribute to the hybrid structure of the molecule.
The actual structure of the sulfur dioxide molecule has partial negative charges on the oxygen atoms and a partial positive charge on the sulfur atoms.
Likewise, the bond order is equal to 1.5, which implies that the bonds between the three atoms are identical intermediates between a single and a double bond.

