Question #8ce42
1 Answer
Jun 27, 2015
Actually, arsenic has 5 electrons in its outermost shell.
Explanation:
Arsenic,
This means that a neutral arsenic atom has 33 electrons surrounding its nucleus. The electron configuration for arsenic looks like this
or, using the noble gas shorthand notation
As you can see, the outermost shell is the fourth shell,
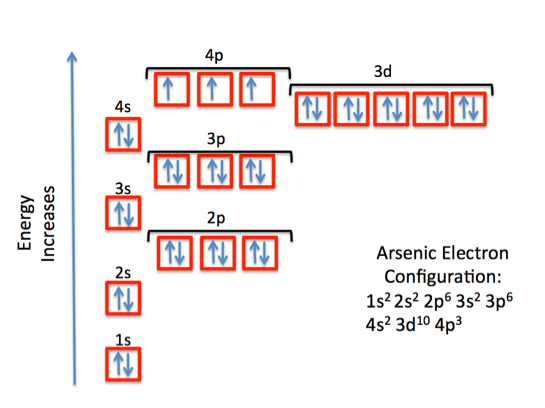
Since arsenic is not a transition metal, its valence electrons will be those electrons located in its outermost shell

