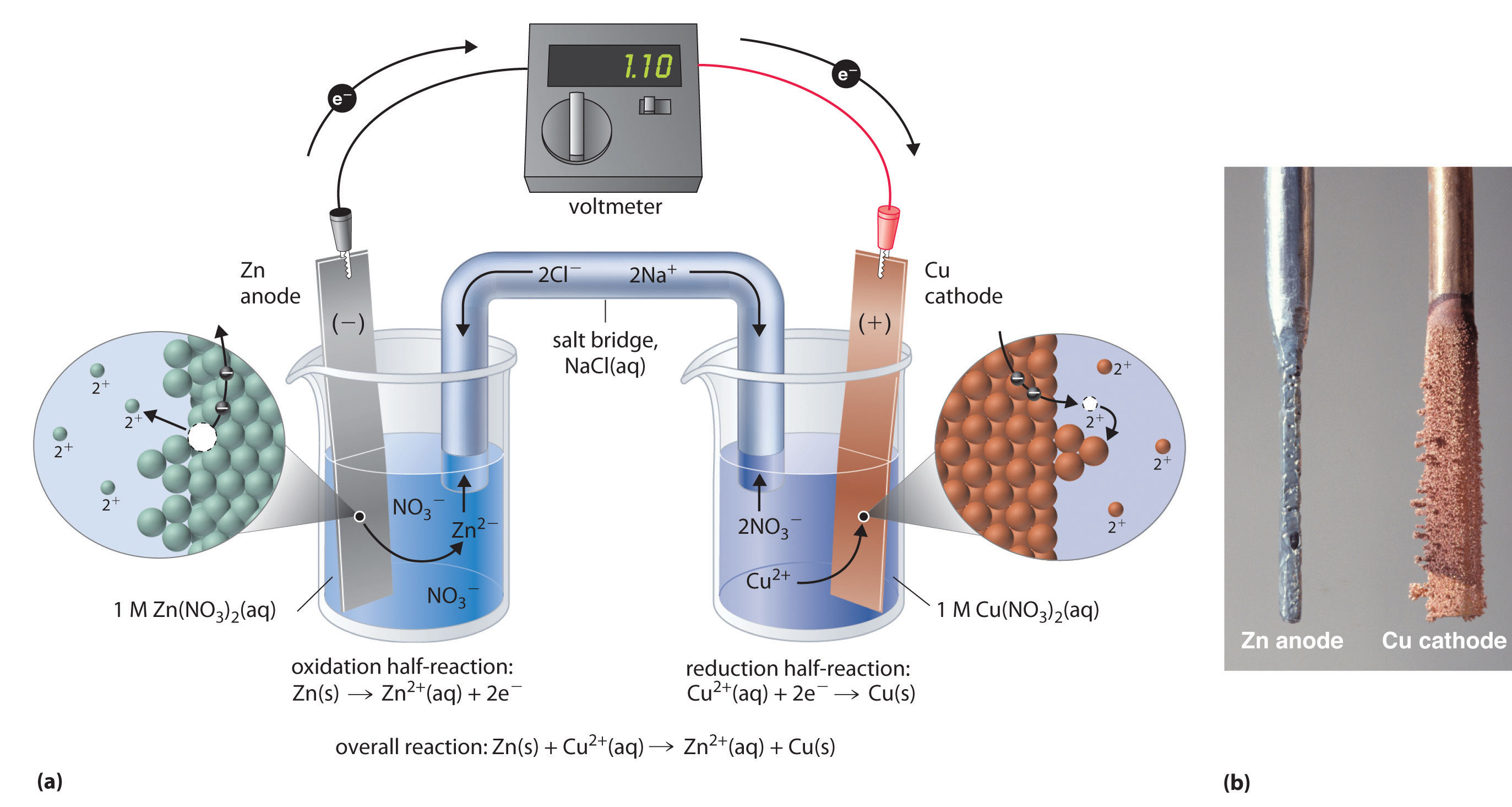
Can you describe the process that releases electrons in a zinc copper voltaic cell?
1 Answer
Electrons are being released because zinc is being oxidized.
Explanation:
A galvanic cell consists of two half-cells, each containing a metal cathode immersed in a solution of its cations, connected via a salt bridge.
In your case, the two half-cells contain a zinc electrode and a copper electrode, respectively.
These electrodes are immersed in copper sulfate, in the case of the copper electrode, and zinc sulfate, in the case of the zinc electrode.
When metals are placed in aqueous solution, they tend to lose electrons to form cations, or positively charged ions. The tendency of a metal to lose electrons depends on its reactivity.
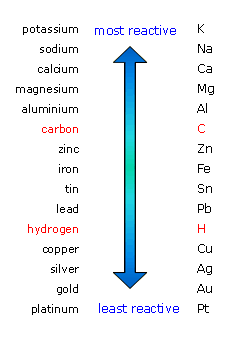
The more reactive a metal is, the easier it will lose electrons. On the other hand, metals that are not particularly reactive will not tend to lose electrons to form cations.
In your case, zinc is more reactive than copper, which means that it will lose electrons more readily. The redox equilibria that govern this galvanic cell are
and
The standard electrode potentials,
The negative
The positive
Therefore, when you connect these two half-cells, the copper will oxidize zinc. Electrons will thus tavel through the wire from the zinc anode to the copper cathode.
As more solid zinc is oxidized to