Question #43d9c
1 Answer
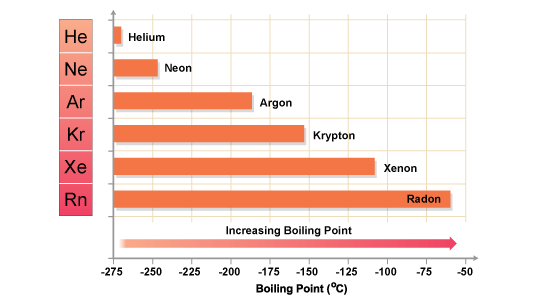
Xenon has a higher boiling point than neon because it exhibits stronger intermolecular forces.
Explanation:
Noble gas atoms only exhibit weak London dispersion forces, which is why their boiling points are so low to begin with.
The strength of the intermolecular forces that exists between a substance's molecules (or atoms) determines how high the boiling point of that substance is
- stronger intermolecular forces
#-># higher boiling point; - weaker intermolecular forces
#-># lower boiling point.
London dispersion forces are the weakest type of intermolecular forces. They are caused by random distortions in the electron cloud of an atom.
When such distorsions are formed, one side of the atom develops a partial negative charge, and the other side a partial positive charge.
These partial charges will then polarize a neighbouring atom's electron cloud, i.e. they will cause another atom to develop partial charges.
This implies that the strength of these partial charges depend directly on the how easily an atom's electron cloud can be distorted.
In your case, xenon has a much bigger electron cloud than neon. Moreover, xenon's outermost electrons are further away from the nucles, which implies that they are not held as tightly as neon's electrons.
As a result, the instantenous distorsions in xenon's electron cloud will produce more significant partial charges, which in turn will determine stronger London dispersion forces to be formed.
Therefore, xenon's boiling point will be higher than neon's.
So, the take-home message is

