How does the metal reactivity series work?
1 Answer
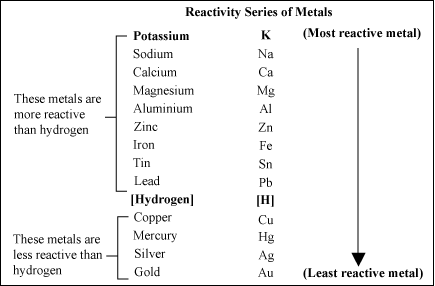
The metal reactivity series lists metals in order of their reactivity, from highest to lowest from top to bottom. A metal listed above another metal will replace that metal in a single replacement (single displacement) reaction.
Explanation:
Example 1.
Does the following reaction occur?
Answer.
Yes. Magnesium is above copper on the reactivity series of metals. Therefore, it will replace the copper in the copper chloride, producing magnesium chloride and solid copper.
Example 2.
Will the reverse reaction occur?
Answer.
No. Copper is below magnesium on the reactivity series of metals, so it will not replace magnesium.
Therefore,