Metal Activity Series
Key Questions
-
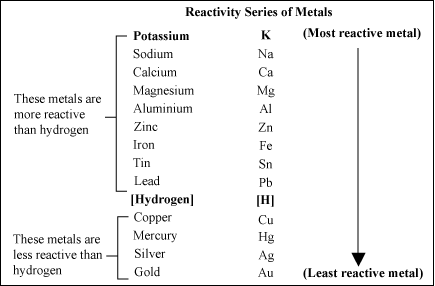
The activity series allows us to predict whether a metal displacement reaction will occur.
You can read about the metal activity series at
http://socratic.org/questions/what-are-metal-activity-series
We can use the series to predict whether a metal displacement reaction will occur.
For example, zinc is above copper in the series. We predict that placing a strip of zinc metal in a copper(II) sulfate solution will produce metallic copper and zinc sulfate.
Copper is below zinc in the series. We predict that a strip of copper placed into a zinc sulfate solution will not produce a reaction.
-
Answer:
The metal reactivity series lists metals in order of their reactivity, from highest to lowest from top to bottom. A metal listed above another metal will replace that metal in a single replacement (single displacement) reaction.
Explanation:
Example 1.
Does the following reaction occur?
#"Mg(s)"+"CuCl"_2("aq")# #rarr# #"MgCl"_2("aq")+"Cu(s)"# Answer.
Yes. Magnesium is above copper on the reactivity series of metals. Therefore, it will replace the copper in the copper chloride, producing magnesium chloride and solid copper.Example 2.
Will the reverse reaction occur?
#"Cu(s)"+"MgCl"_2("aq")# #rarr# #"CuCl"_2("aq")+"Mg(s)"# ?Answer.
No. Copper is below magnesium on the reactivity series of metals, so it will not replace magnesium.
Therefore,#"Cu(s)"+"MgCl"_2("aq")# #rarr# #"no reaction"#